Remarkable alkaloids from Aspergillus versicolor and Ervatamia divaricata
Two recent papers published in The Journal of Organic Chemistry report alkaloids isolated from a fungus culture and from the roots of the tree Ervatamia divaricata.
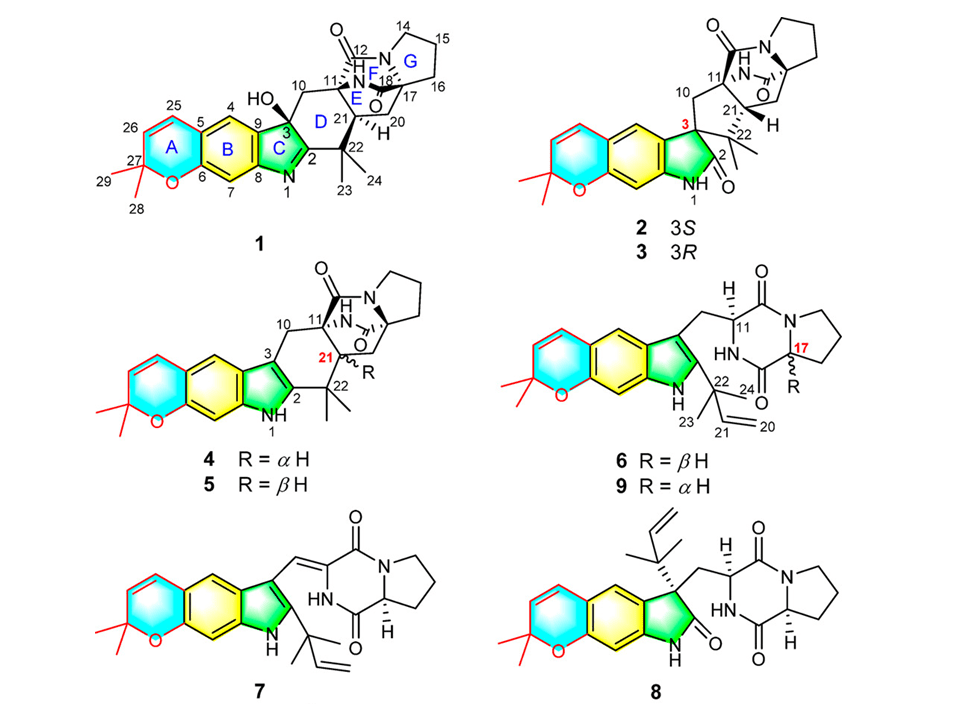
The first paper comes from the groups of Professors Xue, Zhu and Zhang of Huazhong University of Science and Technology. It reports the isolation of 8 new alkaloids from cultures of Aspergillus versicolor. An unusual feature observed for the congeners is the cyclization of a prenyl group, leading to a pyrano[3,2-f ]indole, a rather rare feature observed for prenylated alkaloids. The structures of four of the eight new compounds have been established by X-ray diffraction analysis. The remaining four analogues by NMR and MS analyses. Stereochemical assignments were performed by analysis of NOESY and electronic circular dichroism data. The absolute stereochemical proposition for compound 6 was based on experimental and theoretical [∝]D measurements. Robust spectroscopic data supported the structural assignments.
The alkaloids were tested as inhibitors of nitric oxid production induced by lipopolisaccharide. Compound 7, the most active one, was almost 50 times less active than the control. Structure identification came as the strongest feature of the paper. These alkaloids belong to the class of prenyl-indole alkaloids, whose biosynthesis has been unveiled mainly by David H. Sherman group at the University of Michigan.
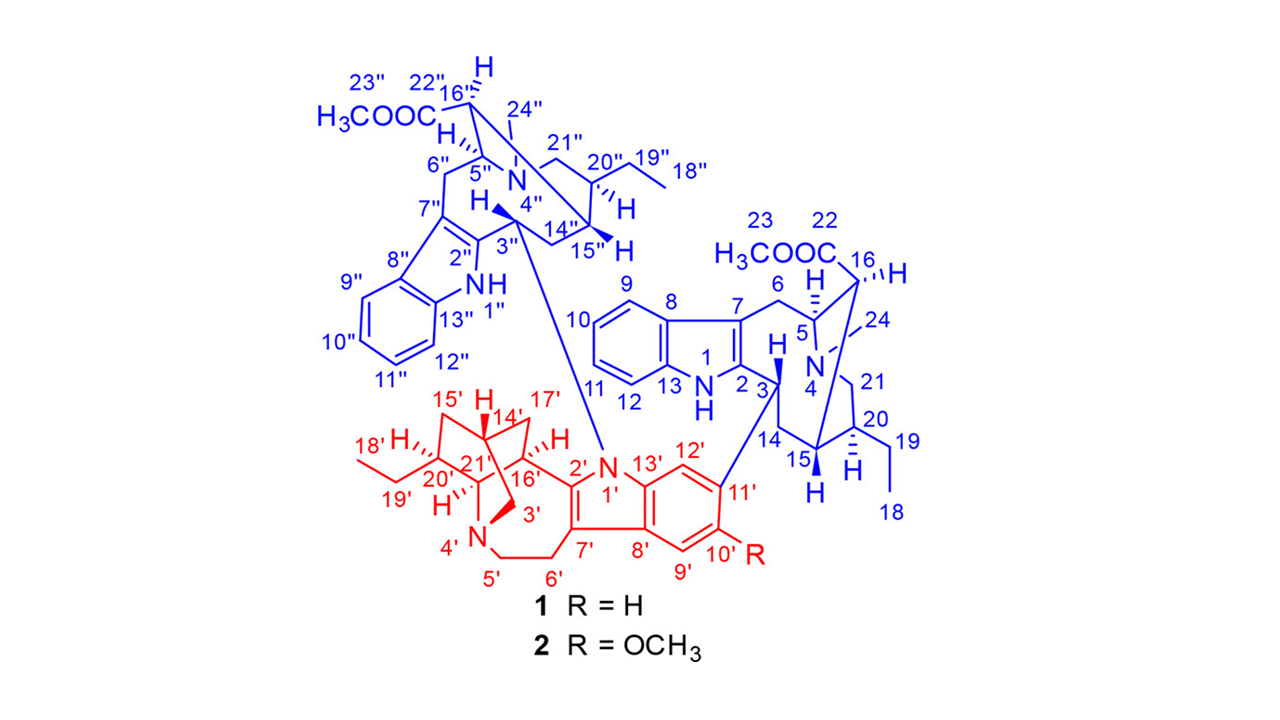
O segundo, do grupo do Prof. Zhang da Jinan University e do grupo dos Profs. Ye e Zhang da Universidade de Macau, reporta as estruturas de dois alcaloides indólicos monoterpenoídicos triméricos. As ervadivaminas A e B foram obtidas a partir de 51,5 kg de raízes de Ervatamia divaricata. Foram isolados 6,0 e 5,0 mg dos alcaloides, em rendimento de aproximadamente 0,0001%. As estruturas dos composto 1 e 2, incluindo configuração absoluta, foram estabelecidas por difração de raios-X e análises de RMN e espectrometria de massas. O alcaloide 1 apresentou atividade citotóxica moderada contra linhagens de células tumorais A-549, MCF-7, HT-29, e HepG2/ADM. Já o alcaloide 2 foi inativo. Novamente, o ponto forte do artigo é o isolamento e determinação estrutural de alcaloides complexos o suficiente para dar muita dor de cabeça a químicos orgânicos sintéticos. Todavia, a ciência do artigo é considerada irrelevante e pouco inovadora.
É urgentemente necessário avisar o editor do Journal of Organic Chemistry que a revista está aceitando para publicação artigos de ciência irrelevante e pouco inovadora.
Refs
Huaqiang Li, Weiguang Sun, Mengyi Deng, Qun Zhou, Jianping Wang, Junjun Liu , Chunmei Chen, Changxing Qi, Zengwei Luo, Yongbo Xue, Hucheng Zhu, and Yonghui Zhang. Asperversiamides, Linearly Fused Prenylated Indole Alkaloids from the Marine-Derived Fungus Aspergillus versicolor. J. Org. Chem., DOI: 10.1021/acs.joc.8b01087.
Zhi-Wen Liu, Jian Zhang, Song-Tao Li, Ming-Qun Liu, Xiao-Jun Huang, Yun-Lin Ao, Chun-Lin Fan, Dong-Mei Zhang, Qing-Wen Zhang, Wen-Cai Ye, and Xiao-Qi Zhang. Ervadivamines A and B, Two Unusual Trimeric Monoterpenoid Indole Alkaloids from Ervatamia divaricata. J. Org. Chem., DOI: 10.1021/acs.joc.8b01371.
